Medical Devices / In Vitro Diagnostics
Let the AMS Medical Device Expert group
help you generate solutions:
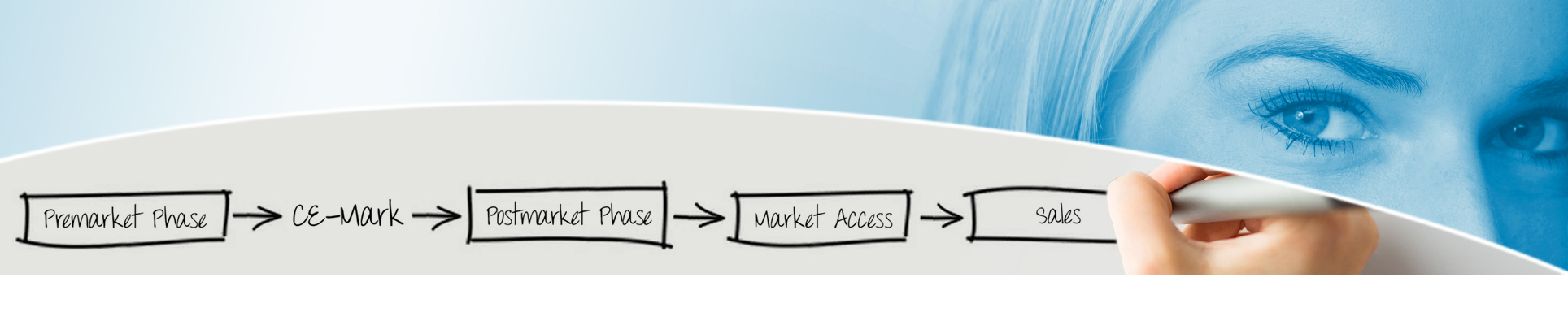
Get premarket or postmarket phase aid for:
- Classification
- Regulatory tasks
- Planning and conduct of clinical investigations / clinical performance studies around your product
- Medical Device Vigilance