Monitoring - read more
AMS with its own permanently employed Clinical Research Associates across Europe and its partner CROs, globally, achieves extraordinarily good results in many aspects. This has been confirmed repeatedly by our clients.
AMS CRAs have a broad range of indication experience with a strong focus in oncology and immunology (among others). Specialized teams operate with specific processes in early development clinical trials or late stage projects, respectively.
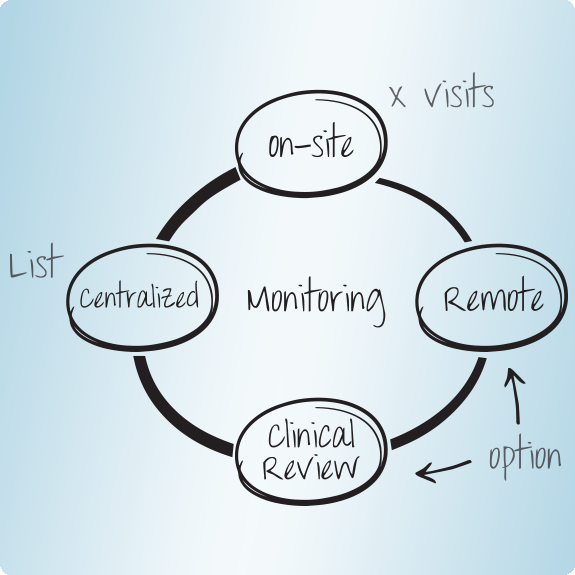
AMS over the last decade repeatedly was the 1st to introduce new monitoring techniques and benefits today from having sound experience in using processes, such as Remote Monitoring, Centralized Monitoring and Risk-based-Monitoring.
AMS operates a fully validated and easy to use EDC-system with extraordinarily good user acceptance at site level. Additionally, AMS provides the most modern BYOD ePRO technologies* which have opened new possibilities in data collection, directly derived from patients.
*the fully validated AMS BYOD-type Patient Reported Outcome solution (AMS -ePRO;) will help to save money and time, e.g. with Patient Diaries and Quality of Life Questionnaires. It significantly reduces the administrative burden in clinical trials.