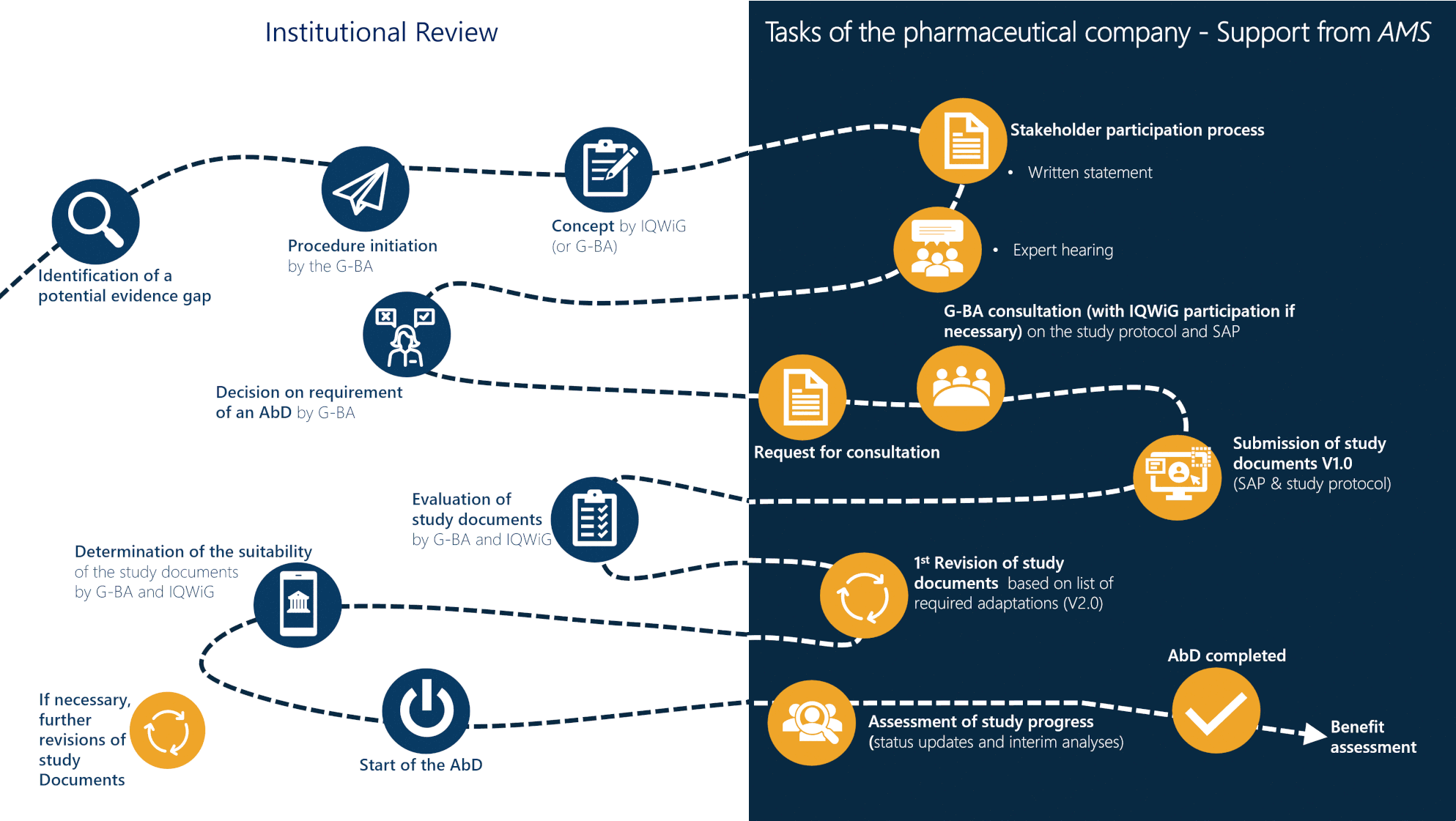
AMNOG benefit assessment
The successful placement of your product on the market is significantly influenced by a professional implementation of all components of the early benefit assessment according to AMNOG. The way to the additional benefit of your medication compared to the standard therapy leads you through the tension between IQWiG, G-BA and the National Association of Statutory Health Insurance Funds (GKV-SV).
Started as pioneers in 2010, AMS is now one of the market leaders in the field of AMNOG benefit assessment. An interdisciplinary team of medical writers and statisticians accompanies you professionally through the entire benefit assessment process. In addition to AMNOG benefit assessments, we are also happy to support you in the area of EU HTA.
Our services
- (Early) G-BA consultation
You will receive support with the (early) G-BA consultation regarding the strategic selection and formulation of questions on study design, appropriate comparative therapy, etc. - Strategic formulation of the benefit dossier
Depending on your needs, we prepare all five modules of your benefit dossier or just individual sections. In close cooperation with you, we develop a targeted value story and, based on this, the best suitable strategy that runs through the dossier as a common thread. - Systematic research
We would be happy to help you with systematic literature and study register research in accordance with G-BA requirements. - Successful pricing
Our pricing team advices you on pricing and reimbursement policy issues in order to set up a successful pricing strategy together. - Epidemiology & derivation of patient numbers
We support you in deriving and presenting the patient numbers for Module 3 of the benefit dossier. - Strategic endpoint mapping & biostatistical services
The patient reported outcomes (PRO) are the beating heart of the benefit assessment. How can you, as a company, position yourself strategically here? Our biostatisticians can carry out all relevant analyses for you. - Written Statement
Together with you we identify possible gaps in the evidence and develop a target-oriented strategy for preparing the written statement. - Simulation of the oral hearing
We offer an individual hearing simulation: you will experience a staged G-BA oral hearing and have the opportunity to practice answering critical questions.





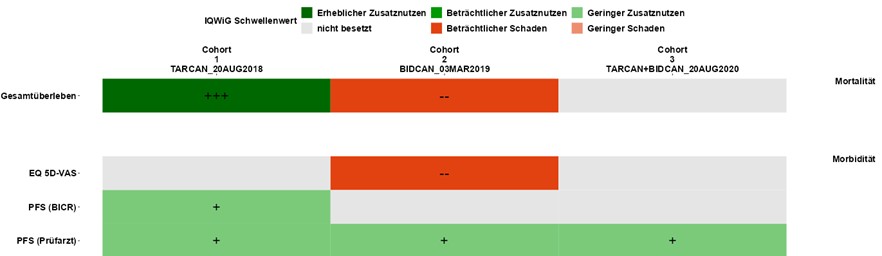
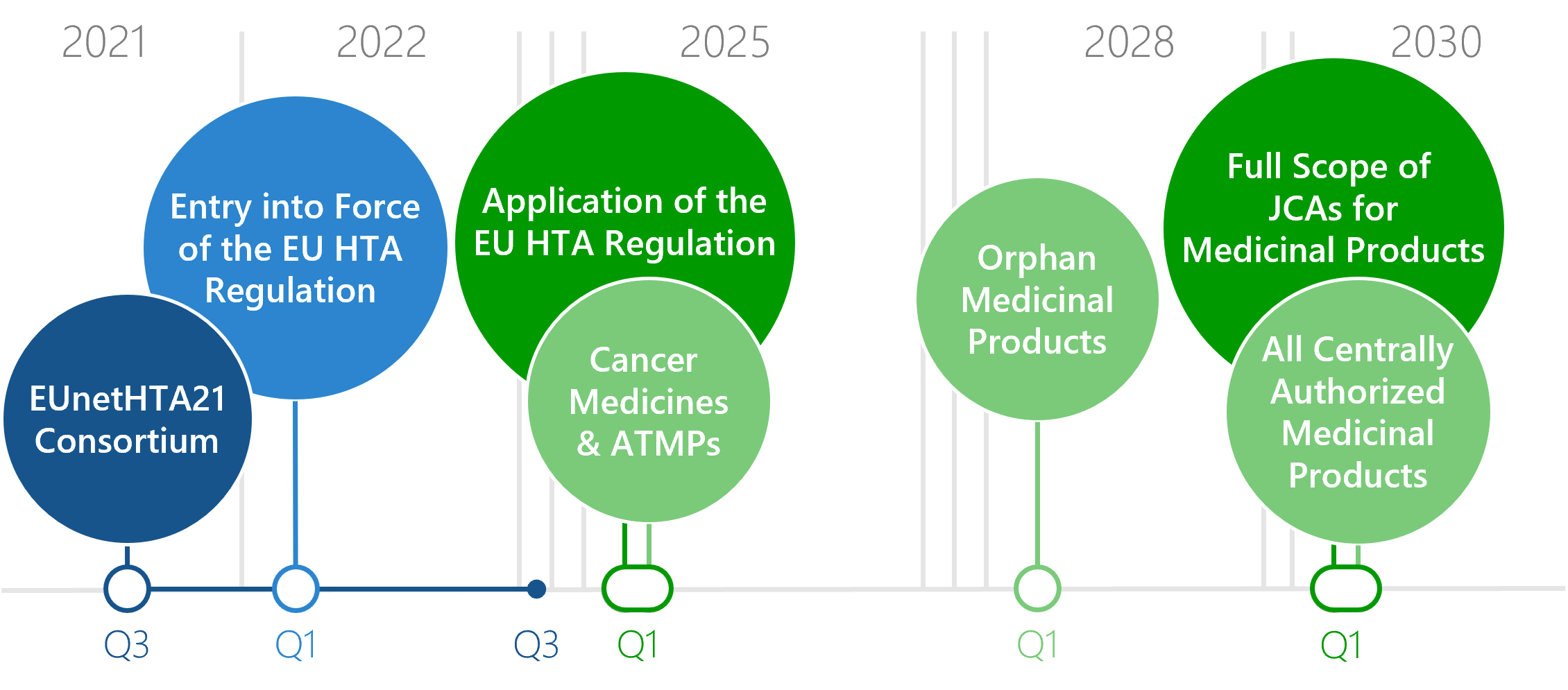
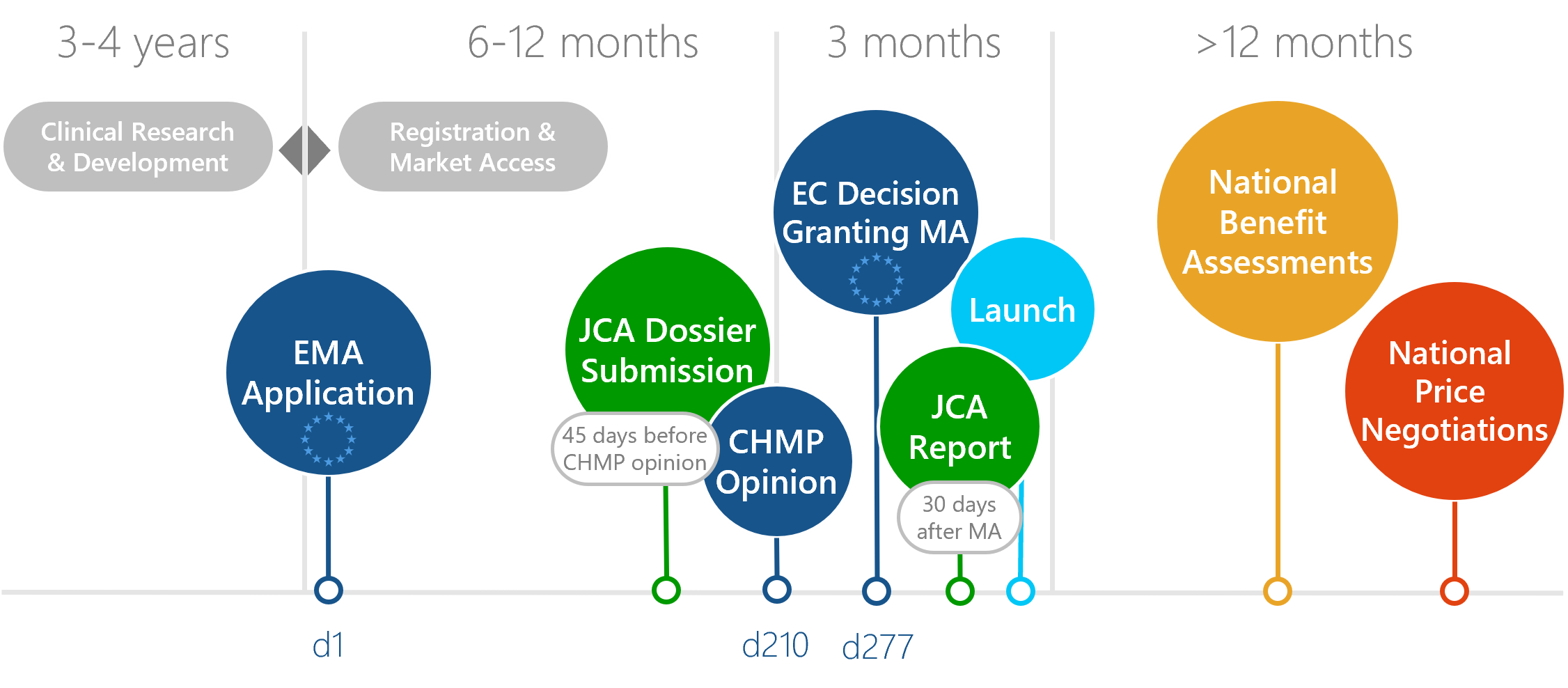 Download:
Download: