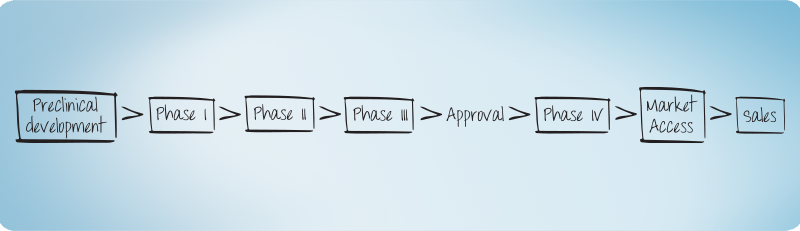
Clinical Development
Starting on the right foot is crucial.
Start your trial with confidence/peace of mind.
An individual view on your clinical trial in the early Phases (Phase I including first-in-man/first-in-patient – adaptive Designs) is essential for successful drug development.
In addition to in depth experience, especially in oncology, you‘ll also find first class international regulatory affairs services at AMS.
Design the most effective trial for quicker approval.
Read more or find out how AMS Clinical Research services can meet your needs: