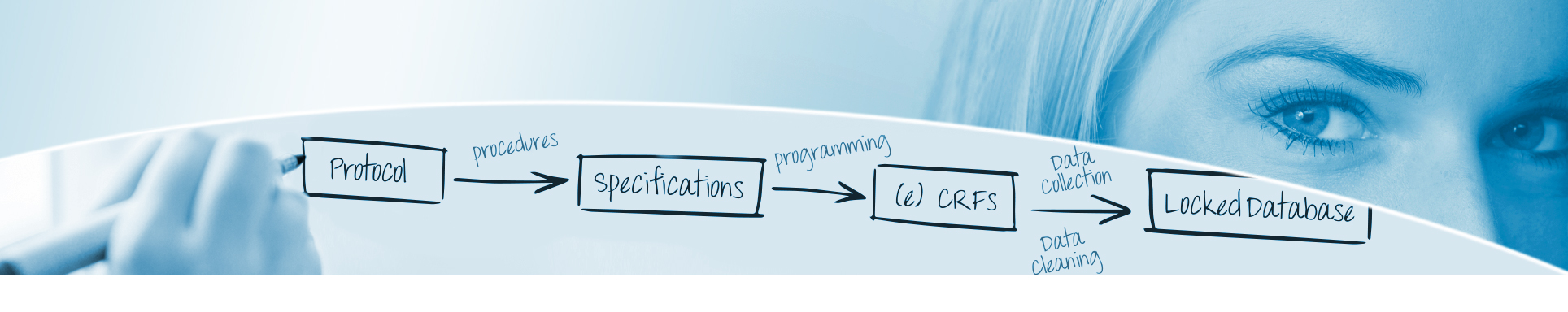
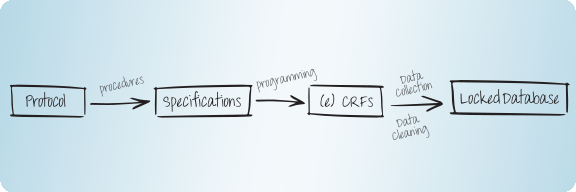
Data Management - read more
AMS follows the CDISC data standards, for the collection of data according to CDASH and for analysis and submission of data according to ADaM and SDTM.
AMS uses the clinical database management system (CDMS) / Electronic Data Capture (EDC) System Clincase ® for both EDC and paper trials. The system offers integrated modules for standard DM status reports, eFeasibility, eLearning and eRandomization.
In addition AMS has developed an electronic Patient Reported Outcome system (AMS -ePRO ) for the online collection of patient questionnaires and patient diaries allowing direct entry by patients themselves using their own devices (any type of smartphones, computers, iPads, etc.).
For coding with MedDRA and WHO Drug Dictionary AMS uses the PACE ® system from Clearight ®.
AMS Validation Managers with in-depth know-how in the field of Computer System Validation (CSV) ensure installation and use of AMS fully validated 21 CFR part 11 compliant systems in Data Management.