DiGA - read more
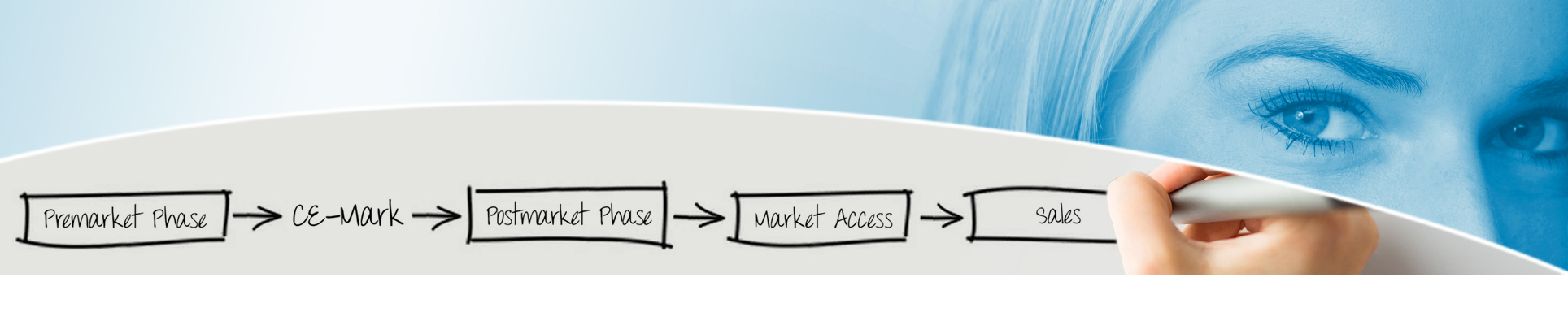
Fast-Track Process for digital health applications
Let the AMS DiGA expert group help you generate solutions:
We support you with our knowledge and experience and pave the way for your health app or web application to receive reimbursement as we did with other industrial partners and software developers with the following services:
- assistance with the consultation at the BfArM Innovation Office prior to application regarding study design and quality requirements
- advisory and regulatory support for successful completion of the digital application forms
- writing the evaluation concept including literature research and systematic data evaluation, and designing the study protocol to detail the planned demonstration of the chosen positive care effects
- conduct of the (randomized) study
- reporting and publishing the results
Please contact for further information on our services for the FAST TRACK procedure: